Venetoclax (Ven) administered daily with pulse-cycled parenteral decitabine (Dec) or 5-azacytidine (5Aza) is standard therapy for acute myeloid leukemia (AML) in the elderly. In practice, toxicity/myelosuppression is frequent, and prompts Ven dose reductions, but by guess-work, because the mechanism downstream of BCL2-inhibition by which Ven augments Dec/5Aza activity is unclear. For the first time, we show that Ven inhibits de novo pyrimidine synthesis, an effect that can guide its integration with Dec/5Aza in a way that enhances anti-AML activity without suppressing normal myelopoiesis.
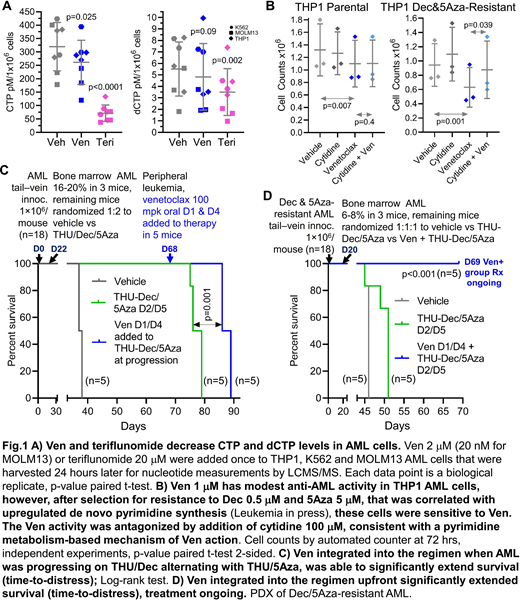
Dec and 5Aza are pro-drugs processed by pyrimidine metabolism into a deoxycytidine analog that depletes the key epigenetic regulator DNA methyltranseferase 1 (DNMT1), a pharmacodynamic effect that terminates malignant but not normal self-replication. We recently demonstrated that Dec- and 5Aza-resistance emerges automatically from adaptive responses of the pyrimidine metabolism network to Dec/5Aza-induced nucleotide perturbations, such that Dec/5Aza processing into DNMT1-depleting nucleotide is forestalled (Leukemia - https://rdcu.be/b58pS). A key element in this auto-resistance is upregulated de novo pyrimidine synthesis, that out-competes salvaged Dec/5Aza. De novo pyrimidine synthesis has an electron-transport dependent mitochondrial step executed by dihydroorotate dehydrogenase (DHODH): BCL2-inhibition by Ven depolarizes mitochondrial membranes - we examined for the first time Ven impact on DHODH/pyrimidine synthesis (others have focused on apoptosis and other metabolic consequences of mitochondrial depolarization). Consistent with Ven inhibiting DHODH/pyrimidine synthesis, both Ven and the direct DHODH inhibitor teriflunomide, at non-apoptotic concentrations, significantly decreased cytidine- and deoxycytidine triphosphate (CTP, dCTP) in AML cells (Fig1A). To see if this effect of Ven can counter Dec/5Aza resistance, we selected for THP1 AML cells double-resistant to Dec 0.5 μM and 5Aza 5 μM - these cells upregulated expression of de novo pyrimidine synthesis enzymes and contained uridine, cytidine and deoxycytidine at levels up to 7-fold higher than parental cells. The double-resistant AML cells were significantly cytoreduced by Ven at a clinically relevant concentration of 1 μM, even though parental THP1 AML cells were minimally sensitive (Fig1B). Consistent with inhibition of de novo pyrimidine synthesis as the mechanism, this action was significantly abrogated by cytidine supplementation (Fig1B). We previously showed that timed alternation of Dec with 5Aza, and incorporation of the cytidine deaminase inhibitor tetrahydrouridine (THU), counters metabolic Dec or 5Aza-resistance to extend non-cytotoxic DNMT1-depletion and survival in vivo (https://rdcu.be/b58pS). However, AML still eventually progresses, via upregulated de novo pyrimidine synthesis. In considering use of Ven to counter this mode of resistance, we reasoned that concurrent administration risks antagonism, because Ven can cause transient cytostasis, and DNMT1-depletion by Dec/5Aza is S-phase dependent. Prior Ven administration, however, creates effect-time sufficient to deplete endogenous nucleotides from AML cells and hence upregulate pyrimidine salvage (that uptakes Dec/5Aza) (Fig1A), as well as resumed cell cycle. Therefore, in vivo in a patient-derived xenotransplant model (PDX) of AML, we introduced Ven (at human equivalent dose), parsimoniously 2X/week, the day before each Dec or 5Aza administration, and at the time of overt AML progression on the base THU-Dec/5Aza regimen. This minimalist Ven application significantly extended survival (time-to-distress) even though it was initiated at progression (Fig1C). As expected, use of this regimen upfront in a PDX of Dec/5Aza-resistant AML produced even greater (several-fold) survival extension (treatment ongoing) vs the base regimen (Fig1D). A non-cytotoxic/non-myelosuppressive mechanism-of-action was confirmed by serial blood counts on-therapy.
In practice, Ven dose in combination with Dec or 5Aza to treat AML is frequently empirically reduced because of toxicity/myelosuppression. Instead, Ven inhibition of de novo pyrimidine synthesis can guide mechanism-based, intermittent administration that avoids toxicity/myelosuppression yet enhances Dec/5Aza anti-AML activity.
Maciejewski:Novartis, Roche: Consultancy, Honoraria; Alexion, BMS: Speakers Bureau. Saunthararajah:EpiDestiny: Consultancy, Current equity holder in private company, Patents & Royalties: University of Illinois at Chicago.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal